A phase 1b randomised controlled trial of a glucagon-like peptide-1 and glucagon receptor dual agonist IBI362 (LY3305677) in Chinese patients with type 2 diabetes
Abstract
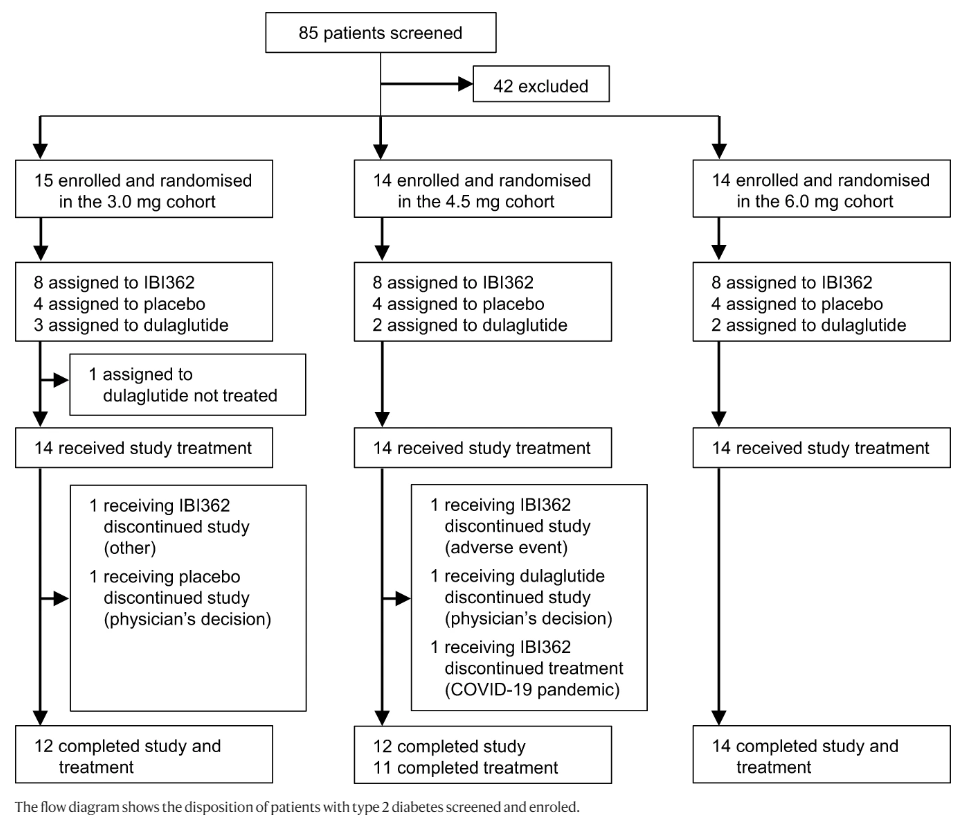
The success of glucagon-like peptide-1 (GLP-1) receptor agonists to treat type 2 diabetes (T2D) and obesity has sparked considerable efforts to develop next-generation co-agonists that are more effective. We conducted a randomised, placebo-controlled phase 1b study (ClinicalTrials.gov: NCT04466904) to evaluate the safety and efficacy of IBI362 (LY3305677), a GLP-1 and glucagon receptor dual agonist, in Chinese patients with T2D. A total of 43 patients with T2D were enrolled in three cohorts in nine study centres in China and randomised in each cohort to receive once-weekly IBI362 (3.0?mg, 4.5?mg or 6.0?mg), placebo or open-label dulaglutide (1.5?mg) subcutaneously for 12 weeks. Forty-two patients received the study treatment and were included in the analysis, with eight receiving IBI362, four receiving placebo and two receiving dulaglutide in each cohort. The patients, investigators and study site personnel involved in treating and assessing patients in each cohort were masked to IBI362 and placebo allocation. Primary outcomes were safety and tolerability of IBI362. Secondary outcomes included the change in glycated haemoglobin A1c (HbA1c), fasting plasma glucose (FPG) and post-mixed-meal tolerance test (post-MTT) glucose levels. IBI362 was well tolerated. Most commonly-reported treatment-emergent adverse events were diarrhoea (29.2% for IBI362, 33.3% for dulaglutide, 0% for placebo), decreased appetite (25.0% for IBI362, 16.7% for dulaglutide, 0% for placebo) and nausea (16.7% for IBI362, 16.7% for dulaglutide and 8.3% for placebo). HbA1c, FPG and post-MTT glucose levels were reduced from baseline to week 12 in patients receiving IBI362 in all three cohorts. IBI362 showed a favourable safety profile and clinically meaningful reductions in blood glucose in Chinese patients with T2D.
Introduction
Type 2 diabetes (T2D), characterized by hyperglycaemia, impairment of insulin secretion and insulin resistance, is a global health crisis with ever-growing incidence and prevalence. An estimated 463 million adults lived with diabetes in 2019, more than 90% of whom had T2D, with a projected increase to 700 million by 2045. China has the largest number of adults with diabetes in 2019 and is estimated to top the list in the coming decades. Often accompanied and exacerbated by obesity and overweight, T2D leads to a series of microvascular and macrovascular complications, which cause profound distress to patients and impose a huge burden on the healthcare system.
State-of-the-art T2D therapeutics, namely glucagon-like peptide-1 (GLP-1) receptor agonists and sodium-glucose cotransporter-2 (SGLT-2) inhibitors, are used either alone or as an add-on to standard-of-care treatment, achieve desirable glycaemic control targets, confer non-glycaemic benefits of body weight loss and blood pressure reduction, and reduce the risk of atherosclerotic cardiovascular disease, congestive heart failure and chronic kidney disease3. Despite the robustness of GLP-1 receptor mono-agonists, the change in gut hormone milieu accompanying substantial glycaemic improvements in patients undergoing bariatric surgery implied that a combination of gastrointestinal hormones may prove additive or even synergistic therapeutic outcomes. Unimolecular poly-agonists with balanced activities at multiple gastrointestinal hormone receptors emerge as the promising next-generation therapeutics for the treatment of T2D and metabolic disorders.
Oxyntomodulin (OXM), a gut hormone that activates both the GLP-1 receptor and glucagon receptor, has been proved to increase energy expenditure while reducing energy intake. The anorectic and energy expenditure-promoting effects of OXM are mediated by activation of GLP-1 receptor and glucagon receptor, respectively. When administered exogenously, OXM can improve glucose tolerance and result in body weight loss, making GLP-1 and glucagon receptor dual agonists a promising treatment option for patients with T2D and/or obesity. However, the inherited stimulation of gluconeogenesis and glycogenolysis by glucagon requires the optimal balancing of GLP-1 and glucagon receptor agonism. Indeed, several dual agonists of this class have yet to demonstrate satisfactory efficacy on both glucose reduction and body weight loss, albeit with overall favourable safety profiles.
IBI362 (also known as LY3305677) is a once-weekly synthetic peptide analogue of mammalian OXM, with a fatty-acyl moiety to extend the half-life. IBI362 potently bound to human and mouse GLP-1 receptors and glucagon receptors in vitro. In mice, IBI362 improved glucose control, decreased body weight in both Gcgr knockout (KO) and Glp1r KO settings and increased energy expenditure. Furthermore, a first-in-human single-ascending-dose study in healthy subjects and a multiple-ascending-dose study in Chinese adults with overweight and obesity demonstrated the favourable safety profile, weight loss efficacy and multiple metabolic benefits of IBI362.
Here, in a randomised, placebo-controlled, multiple-ascending-dose phase 1b study, adopting the same dose regimens as the previous phase 1b study in Chinese participants with overweight or obesity, we evaluate the safety, tolerability, pharmacokinetics and efficacy of IBI362 in Chinese patients with T2D. IBI362 is well-tolerated, shows an overall favourable safety profile and demonstrates clinically meaningful glycemic control and weight loss, as well as multiple metabolic improvements in Chinese patients with T2D.
For More information, please visit: https://www.nature.com/articles/s41467-022-31328-x#Fig1
sources:Nature Communications, (2022) 13:3613
Published: 24 June 2022
DOI: 10.1038/s41467-022-31328-x
Disclaimer:
Partial content of this page is transferred from the network, only for the use of scientific communication, if there is infringement, please contact us to delete. See the Privacy Policy for more information.