Dispersion of asphaltenes and paraffins in crude oil using Multicationic ionic liquids
Ref. No. SSTCRC015
Background:
Asphaltene precipitation is one major problem in crude oil production and in petroleum industry. Therefore, various methods have been used to solve this problem.
It is important to mention here that, to the best of our knowledge, no work has been done in dispersion of asphaltenes and paraffins from crude oil with Multicationic surface active ionic liquids (SAILs). Ionic liquids are well known with prominent properties. Among them surfactant behavior and multi branch structure, i.e. Multicationic, are outstanding.
The influence of conventional surfactants and single-chain ionic liquids has been reported. Below is a brief description of two relevant recently used investigations.
The effect of imidazolium-based single-chain SAILs in preventing asphaltene precipitation has been reported. For this purpose, a polymer was used as a carrier single-chain SAILs with different chain lengths and anions. The compounds could prevent the accumulation and deposition of asphaltenes and by forming polar layers around the asphaltene particles, reduce their accumulation. The amount of precipitation was reduced by 50% and the size of asphaltene particles by 80% as documented in: [Dong, He, et al. "Inhibition of asphaltene precipitation by ionic liquid polymers containing imidazole pendants and alkyl branches" Energy and Fuels 36(13) 2022: 6831-6842].
In another study the effect of a series of pristine single-chain ionic liquids to prevent precipitation of asphaltenes has been studied. The surfactants were with different alkyl chain and different anions. The synthesized ILs were evaluated as asphaltene dispersants based on two methodology. Using viscometric criterion, it was shown that the asphaltene onset precipitation was at 28.5 vol%. However, this percent was postponed to 42.8, 50, 78.5, and 64.3 vol.%, by adding the SAILs. The other criterion to follow the results was spectroscopic analysis. It was confirmed that low amounts of remaining deposits had smaller particle size and the crude oil fluid was with a low viscosity. [Ghanem, Alaa, et al. "Synthesis and characterization of imidazolium-based ionic liquids and evaluating their performance as asphaltene dispersants" Materials 15(4) 2022: 1600].
Accumulation and precipitation of high molecular weight asphaltenes and paraffins are important issues in the oil recovery processes. These phenomena cause serious economic problems by clogging wells, pumps, valves, and pipes through oil production and transportation, which reduces extraction and, in some cases, completely stops the oil production.
The stability of asphaltene and paraffin aggregates are attributed to the coexisting of these molecules with polar substances such as resins, which form micellar like associations in petroleum industry. As is shown in Figure 1 (a), the asphaltene molecules self-associate in micellar core while resins attach them at polar heads and stretch their aliphatic branches outward to form a steric-stabilization layer around the asphaltene molecules. The same arrangement would be possible with paraffins. A common way for dissolving these aggregates is using aromatic solvents. Hence, it is a costly method and time-consuming procedure.
Surface active ionic liquids, briefly SAILs, can break the asphaltene and paraffin associations due to amphiphilic nature. Contrary to some conventional surfactants SAILs can highly maintain their influence under harsh temperature and salinity conditions. Figure 1 (a) shows that SAILs adsorb via the nonpolar alkyl chain on the resin-stabilized layers and set their polar head groups outward, creating a polar shell surrounding the aggregates. Then the repulsion forces between the polar shells banish the asphaltenes and paraffins associations from each other. Thus SAILs could dissolve precipitates.
Different studies indicate that, in addition to the surface active ionic liquids (SAILs) interactions with the resin-stabilized layer, the formed aggregates can interact with each other and create either loose or tight new assembles, while the tight assembles are not desired (Figure 1.b). Considering this, SAILs that interact strongly with resin-asphaltene aggregates, but exhibit slight affinity (or nothing at all) to themselves are preferred. In this regard, SAILs with medium alkyl chain and located charge head groups seem to be preferred.

Figure 1: A scheme of an asphaltene-resin structure (a) and loose or tight assembles of asphaltene-resin- SAILs (b).
On the other hand, as shown in Figure 2, by adsorbing SAILs on the surface of asphaltene particles, due to the polarity of SAILs, asphaltenes are also polarized and dispersed in the aqueous phase. Then, the electrostatic repulsion between the created masses, prevents the asphaltene particles from sticking together and re-depositing.
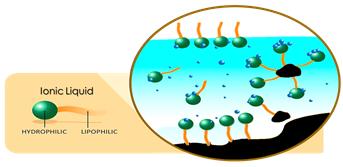
Figure 2: A scheme of the adsorption of SAILs on the asphaltene particles and their dispersion in the aqueous phase
Meanwhile, Emulsions of water-crude oil are mostly stable due to forming a rigid interfacial asphaltene film (Figure 3.a). Asphaltenes usually tend to accumulate at the interface by forming a thin and continuous film and create stabile water-crude oil emulsions. Accordingly, SAILs have been found as suitable additives for dehydrating and desalting crude oils. Figure 3(b) shows that by the migration of SAILs into the interface, the interfacial asphaltene films become disrupted, which leads to emulsion rupture and phase separation. Finally, SAILs can assist phase transition by reducing the IFT between water and oil.
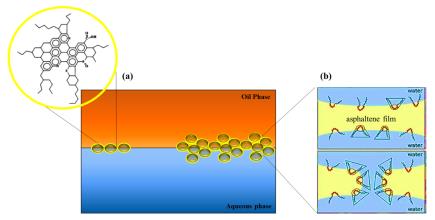
Figure 3: Typical asphaltene molecules and their interfacial arrangement (a) and emulsion rupture with SAILs (b).
Molecular tailoring can bring about the desired properties and efficiency of these materials. To increase the effectiveness, SAILs with higher hydrophobic properties are needed for high adsorption level on the surface of asphaltenes and, as a result, low deposition of asphalentenes. In this regard, a new category of SAILs, known as "Multicationic" including Gemini and Tripodal has been proposed. Single-chain SAILs consist of only one polar segment and one non-polar alkyl chain and If two single-chain ionic liquids are joined by a spacer group, a Gemini SAIL molecule is made. Also, by connecting three polar parts with three non-polar groups, the Tripodal SAILs can be produced. Single-chain, Gemini, and Tripodal SAILs are shown in Figure 4.
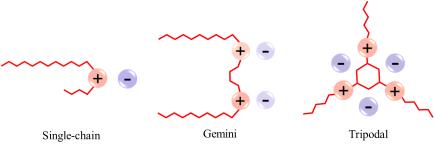
Figure 4: Single-chain, Gemini, and Tripodal SAILs.
Our previous studies, indicated in many publications, indicate that Multicationic SAILs are more effective than conventional surfactants and single-chain SAILs in reducing interfacial tension, the critical micelle concentration (CMC), contact angle of oil phase with solid surfaces and in altering wettability from oil-wet to water-wet. Their performance in interfacial tension reduction and in CMC has been revealed as half or one third of that obtained with single chain SAILs. The reason for the better performance of Multicationic SAILs is their unique structure. This class of SAILs has a higher interfacial activity due to having several hydrophilic and hydrophobic groups in addition to the spacer group which regularize the placement of SAIL molecules at the interface and reduces the repulsion of their charges.
In the light of these results and considering what has been revealed with single chain SAILs in dispersing asphaltenes (pointed above), a much higher performance is expected by using Multicationc SAILs in the important case of asphaltene dispersion. These materials tend to be more adsorbed on the surface of asphaltene particles/deposited surfaces, resulting in a significant reduction of accumulations and sediments and keeping them dispersed in crude oils. Adding to these, the several time regenerating and capability of life time under harsh conditions of reservoirs can establish good economic perspective in preventing asphaltene deposits during crude oil production, transportation and exploitation.
In this project, new structure Gemini and Tripod SAILs will be designed and synthesized to examine their efficiency. Believed that SAILs have great potential to act as superior surfactants in the dispersion of asphaltenes and paraffins.
Cooperation Needs:
As the first step of this project, we, in our country, design and synthesize unique Multicationic SAILs, characterize them and evaluate their stability under different temperatures, levels of salinity and pHs. Perhaps some characterizations to be performed in your country to benefit the modern technical instruments.
We have been working with several instruments and utilities to measure interfacial tension, contact angle and wettability of crude oil-water system. However, To start this project, two or three set-ups should be established here in Bu-Ali Sina University. The preliminary outline and required facilities have been designed for manufacturing. These have been according to the standard methods; however, experts in your country/university may help us to have better design and make use of your opinions. Some optic and flow pattern issues are involved in the design considering the procedure of experiments. This part of cooperation seems very useful.
Upon establishing the set-ups, experiments will be performed based on different crude oils available in Iran and data will be generated carefully. The analysis of data and modeling could be the next step of our cooperation to provide a comprehensive investigation.
We may arrange a close visiting of our/your facilities in the both countries/universities and negotiate about better opportunities and better researches while postgraduate students could be involved.
Benefits:
- The project will bring a new technology that can assist the asphaltene and paraffin dispersion in crude oil.
- The project will bring a new technology that can enhance crude oil recovery.
The project will bring a new technology that can reduce crude oil viscosity.
Outputs:
The following outputs are expected:
- At least two academic papers
- One technical patents
- Two laboratory products.